1)RCH(OH)CH3
2)RCOCH3
*R denotes H, alkyl or aryl group
In positive tests, the reaction will produce a yellow precipitate of CHI3.
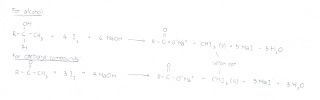
*Click on picture for a clearer and bigger view
Any compound which contains the above mentioned structural units will result in a positive iodoform test. Ethanol is the only first degree alcohol to give a positive iodoform test. The following are some alcohols which may produce reactions with tri-iodomethane.
The compounds which produced a positive reaction all have the specific functional group [RCH(OH)CH3] and the boxed R-group. Propan-2-ol is unable to give a positive reaction because it does not have a CH3.
Here are the some other compounds which may react with tri-iodomethane:

The compounds which produced a positive reaction all have the functional group [RCOCH3] and a respective R-group boxed in red.
To figure out whether a compound has a positive iodoform reaction, always look out for it's functional group : [RCH(OH)CH3] or [RCOCH3]. If the compound does not have the specific functional group, it simply does not give a positive iodoform test and hence the equations for the reaction is not applicable.
-adapted and extended with permission from http://learnsg.blogspot.com-





good
ReplyDeleteYes anaghi this is very good I agree
ReplyDelete