My reply: Okay, firstly i would assume that you're asking about electrophilic addition for both reactions involving Bromine. Bromine as you know is an electrophile, which is also an electron withdrawing atom. Hence, Br in bromoethene will "spread" the electrons out from the electron rich C=C and stabilises bromoethene. In 3-bromopropene (i'm assuming it's 3-bromo-prop-1-ene), the Br atom is too far away from C=C and hence it does not really "spread" the electrons from the pi bond across the molecule. In this way, the C=C pi bond in 3-bromopropene will be more susceptible to a bromine attack compared to bromoethene.
Monday, March 23, 2009
Bromoethene vs Bromopropene
My reply: Okay, firstly i would assume that you're asking about electrophilic addition for both reactions involving Bromine. Bromine as you know is an electrophile, which is also an electron withdrawing atom. Hence, Br in bromoethene will "spread" the electrons out from the electron rich C=C and stabilises bromoethene. In 3-bromopropene (i'm assuming it's 3-bromo-prop-1-ene), the Br atom is too far away from C=C and hence it does not really "spread" the electrons from the pi bond across the molecule. In this way, the C=C pi bond in 3-bromopropene will be more susceptible to a bromine attack compared to bromoethene.
Monday, March 9, 2009
Iodoform test
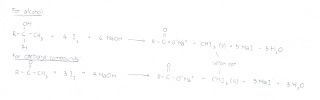
1)RCH(OH)CH3
2)RCOCH3
*R denotes H, alkyl or aryl group
In positive tests, the reaction will produce a yellow precipitate of CHI3.
*Click on picture for a clearer and bigger view
Any compound which contains the above mentioned structural units will result in a positive iodoform test. Ethanol is the only first degree alcohol to give a positive iodoform test. The following are some alcohols which may produce reactions with tri-iodomethane.
The compounds which produced a positive reaction all have the specific functional group [RCH(OH)CH3] and the boxed R-group. Propan-2-ol is unable to give a positive reaction because it does not have a CH3.
Here are the some other compounds which may react with tri-iodomethane:

The compounds which produced a positive reaction all have the functional group [RCOCH3] and a respective R-group boxed in red.
To figure out whether a compound has a positive iodoform reaction, always look out for it's functional group : [RCH(OH)CH3] or [RCOCH3]. If the compound does not have the specific functional group, it simply does not give a positive iodoform test and hence the equations for the reaction is not applicable.
-adapted and extended with permission from http://learnsg.blogspot.com-
Wednesday, March 4, 2009
Iodoform reactions
Ans:
 Okay, i've drawn up the molecules you asked abt and the required structural units for a positive iodoform test. As you can see, both ethanoic acid and ethanamide DO NOT HAVE either of the 2 structural units in the red box. Therefore the iodoform test will present a negative result.
Okay, i've drawn up the molecules you asked abt and the required structural units for a positive iodoform test. As you can see, both ethanoic acid and ethanamide DO NOT HAVE either of the 2 structural units in the red box. Therefore the iodoform test will present a negative result.
Tuesday, March 3, 2009
Area/Volume Formulas
(*included - cuboids, closed cylinder, prism, pyramid, cone, sphere, sector, hemisphere)





